National clinical working groups with doctor, pharmacist and nurse representation from New Zealand providers. Develop nationally agreed and clinically appropriate regimen definitions. Supports nationally consistent data and meaningful analytics.
Anti-Cancer Therapy - Nationally Organised Workstreams
ACT-NOW Programme
The ACT-NOW programme looks at how chemotherapy is being delivered across the motu and will use the national data collected to identify ways chemotherapy can be improved for people with cancer and their whānau.
In New Zealand, people with cancer can experience differences in access to chemotherapy treatment depending on where they live. In order to ensure that all New Zealanders receive the best cancer care, we must be able to compare chemotherapy treatments around the country and use this information to improve the accessibility and quality of treatment. New Zealand has a high-quality health system, but there are always opportunities for improvement. The Anti-Cancer Therapy – Nationally Organised Workstreams (ACT-NOW) programme is about identifying these opportunities within the context of chemotherapy.
ACT-NOW is a priority action under the New Zealand Cancer Action Plan 2019-2029. We want to ensure consistent, high quality, evidence-based and resource efficient care is delivered in all New Zealand cancer centres. ACT-NOW seeks to better understand how chemotherapy is being delivered across the country by collecting treatment and treatment-related data from public and private chemotherapy providers, and working with doctors and other stakeholders, to identify opportunities that can drive real improvements for people with cancer and their whānau.
We have and will continue to support specialist working groups of doctors, pharmacists and nurses, organised by cancer type and representing New Zealand treatment providers. These groups help us understand the data that should be collected for each cancer type. It is important that we focus on collecting the most valuable data in a nationally consistent way so that fair comparisons can be made between District Health Boards (DHBs) and treatment providers.
ACT-NOW is building the technology to securely share the relevant data from hospital information systems to a new national chemotherapy database, where data can be analysed and fed back to key stakeholders in a format that can inform action.
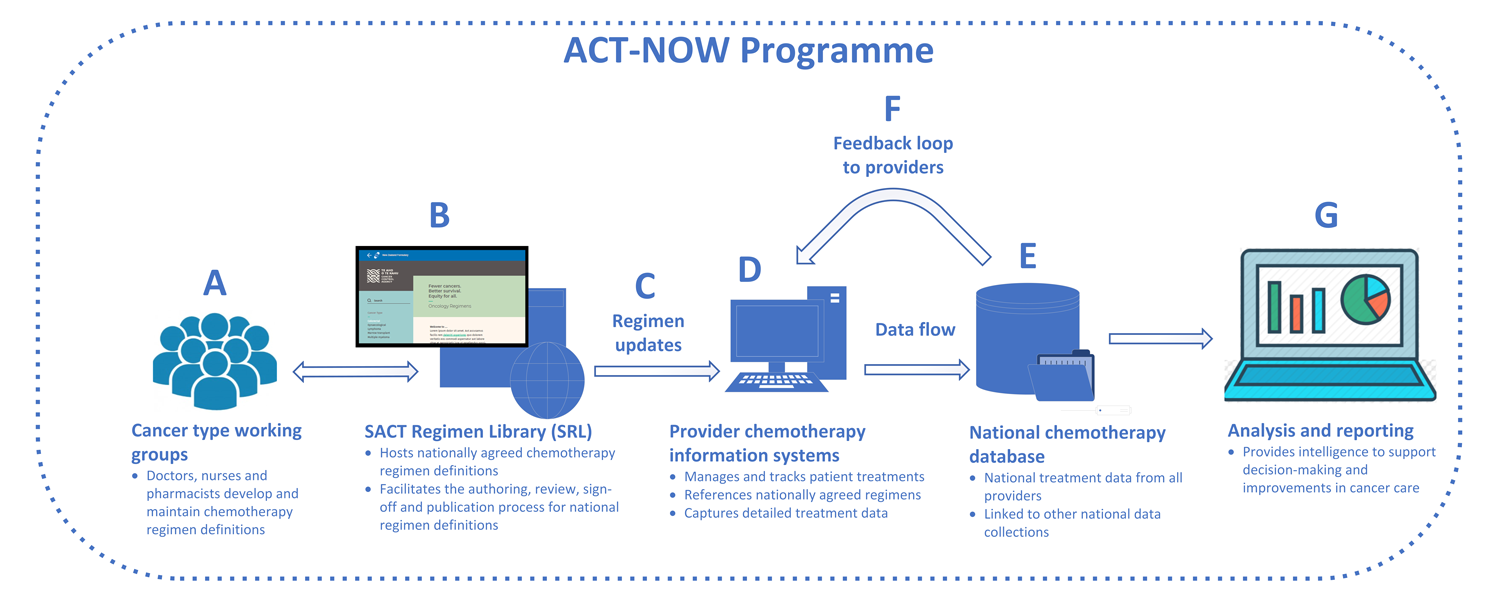
Website to manage the regimen development, maintenance and publication process. This serves as a source of truth for nationally developed regimen definitions.
National regimen definitions are updated within provider electronic systems. SRL makes regimens available in electronic formats to support the automated import and ongoing alignment of regimens within provider electronic systems.
Computer systems to plan and track the treatment of people with cancer. Captures detailed diagnosis and treatment data. Uses treatment regimen definitions from the SRL.
National database of detailed treatment data sourced from provider information systems. Aggregates data from across the country in a single place, ready to be analysed.
Regular data quality reporting back to providers to support ongoing data quality, completeness and alignment, and to ensure that data remains fit-for-purpose.
Regular provision of key information and intelligence back to stakeholders to support improvements to cancer care.
ACT-NOW governance
ACT-NOW is developing processes and governance frameworks to ensure that data are collected, stored, shared and used appropriately to benefit the greatest number of people whilst preserving patient privacy, respecting Māori data sovereignty and preventing inappropriate use of data.
The ACT-NOW programme is governed by the Te Aho o Te Kahu Council, who have commissioned the programme and provide ongoing strategic governance.
Programme sponsorship is through the Chief Executive.
Day-to-day management of the programme is through Te Aho o Te Kahu’s Data Monitoring and Reporting (DMR) group, within which the ACT-NOW programme team sits.
Clinical guidance is provided primarily though the Te Aho o Te Kahu Haematology and Medical Oncology Working Groups (HWG and MOWG respectively), as well as our National (Chemotherapy) Pharmacist, Clinical Lead and Clinical Director.
Key partners in this work include the Ministry of Health, New Zealand Formulary (NZF), Hei Āhuru Mōwai (Māori Cancer Leadership Board), DHBs and the private providers of chemotherapy in New Zealand.
ACT-NOW programme components
There are four broad components to the ACT-NOW programme, each playing a critical role in supporting the programme objectives:
Regimens define the combinations of chemotherapy medicines and supportive care medicines (e.g. anti-nausea medicines) to be given to a person with cancer according to a specific repeating pattern and schedule. Regimens specify the medicines to be used, their dosage, frequency and how they should be administered (e.g. intravenous infusion or oral tablets). The regimens that people receive are important drivers of their cancer outcomes, and so it is important that we are able to collect accurate information on the regimens people are receiving. A current issue is that treatment providers are not naming and describing regimens in the same way, and this makes it difficult to make fair and meaningful comparisons between providers.
The ACT-NOW programme brings representatives from New Zealand cancer centres across different cancer types together in the most ambitious and collaborative national chemotherapy project to date. Under the umbrella of Te Aho o Te Kahu, these groups work together to identify current practice within New Zealand, to review the clinical evidence and to agree common naming conventions and regimen definitions. All chemotherapy regimens commonly used in public and private hospitals are included and any discrepancies are resolved by consensus. Regimen definitions are published in draft format and consulted upon prior to publication in the national regimen library. Expert technical review is undertaken by specialist pharmacists, nurses and doctors to affirm that the regimens are safe and practical within the New Zealand setting. The process supports treatment providers to implement these nationally agreed definitions within their local information systems, which provides a solid foundation for national treatment data to be collected and used with confidence.
Regimen development is prioritised by the cancers that disproportionately affect Māori and that also affect the largest number of people each year. Bowel, lung, breast and prostate cancers (known as the ‘big four’) have been the initial priority. There are approximately 20 different workstreams representing almost all types of cancer. Our goal is to develop national regimen definitions across these 20 streams during 2021/2022.
Nationally agreed regimen definitions must be easily accessible to those who are working with them, including doctors, nurses and pharmacists. The national regimen library will host the definitions on a newly developed website known as the Systemic Anti-Cancer Therapy (SACT) Regimen Library (SACT Regimen Library or SRL). SACT refers to the use of medicines for the treatment of cancer. This includes chemotherapy agents (cancer drugs) used to treat cancer. SACT also includes targeted therapies designed to act on specific molecular targets, immunotherapy to help the immune system, and supportive care medicines to reduce the side effects of treatment (e.g. nausea).
A process has also been developed to regularly review regimens and keep them up to date so that they can evolve in response to new medicines becoming available or changes in clinical practice. The SRL currently hosts agreed regimens for the ‘big four’ cancers: bowel, lung, breast and prostate. During 2021/2022 the SRL will host regimens for most cancer types, including the key supportive care medicine components of these regimens.
We are also working with treatment providers to implement these definitions within the electronic systems they use to plan and track treatments for people with cancer. This will ensure that doctors are using the same set of regimen definitions when prescribing treatments for people with cancer, supporting national consistency and developing a foundation for national data collection.
It is important that we collect the right data to answer the right questions. We need to work closely with medical professionals and other stakeholders including Māori, patients and their whānau, PHARMAC, DHB resource planners and service managers to understand exactly what we should be measuring and how this measurement will contribute to improvements in cancer care, equity and the management of capacity and demand.
Developing a prioritised set of measurement and analysis requirements allows us to generate a detailed understanding of exactly which data need to be collected. We can then assess the feasibility of collecting data in a nationally consistent and complete way.
These requirements are likely to evolve over time as new priorities emerge. ACT-NOW will seek to establish a flexible process to accommodate such changes so that the system is sustainable, remains relevant and can deliver the greatest benefit possible.
With a clear understanding of what we need to measure and the data we need to collect, we can design and build the technology to extract the data from hospital IT systems and load into a new national chemotherapy database. This data can be linked to other national data collections held by the Ministry of Health, including data on cancer diagnostics, surgery, radiation therapy and patient outcomes to further understand the role of chemotherapy within the broader context of cancer treatment and outcomes.


